TEAM RITM
Radiopharmaceuticals, Imaging, Theranostics and Multimodality
Scientific context and objectives
The RITM team develops and evaluates novel radiopharmaceuticals, mainly based on radiometals. These compounds are designed within the framework of a theranostic approach and aim to improve patients’ early diagnosis and therapy.
To this end, the RITM team is developing new molecular tools and methodologies for optimizing the various components of a radiopharmaceutical (vector, chelator, spacer, bioconjugation linker, etc.) and evaluating them in vitro and in vivo. RITM members’ skills range from coordination chemistry to peptide chemistry, from bioconjugation to radiochemistry, from radiopharmaceuticals to preclinical nuclear imaging.
Team members

Head : Dr. Victor GONCALVES (MCF)
- Dr. Bertrand COLLIN (PUPH)
- Pr. Franck DENAT (PR)
- Dr. Sophie POTY (CPJ, UBE)
- Dr. Ibai VALVERDE (CR CNRS)
- Dr. Michael CLARON (IE CNRS)
- Cécile DESINGLE (Tech UBE)
- Dr. Mathieu MOREAU (IE UBE)
Research topics
Topic 1: Molecular tools for the design of new radiopharmaceuticals

Find out more about our 3 research topics :
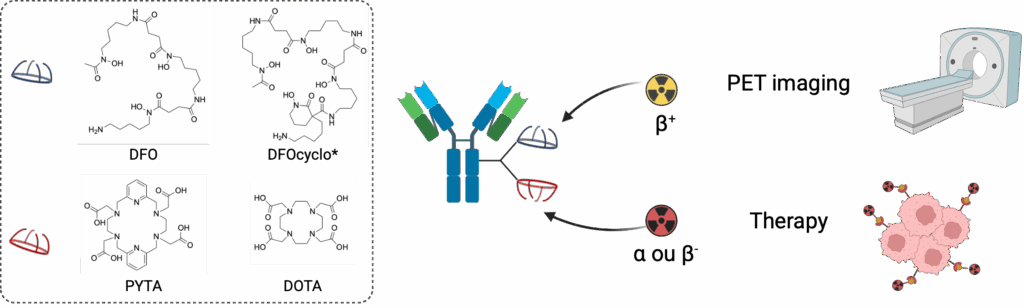
Chelating agents and radiolabeling
Bifunctional chelating agents are a key element in the design of radiopharmaceuticals. Introduced onto a targeting agent, they ensure the stable in vivo coordination of metal radioisotopes. The team’s internationally recognized expertise in this field has led to the creation of the CheMatech spin-off. This research is continuing with the development of new bifunctional chelating agents with optimized properties. This work focuses in particular on chelating agents capable of forming thermodynamically stable and kinetically inert complexes with “theranostic couples” such as 89Zr/177Lu, 89Zr/225Ac or 64Cu/67Cu at room temperature. In this approach, the radiotracer serves as a “diagnostic companion” to the therapeutic counterpart. Another part of our research activities focuses on the design of chelating agents for α-emitting radiometals of growing interest for targeted radionuclide therapy (TRT), such as 225Ac, 212Pb, 227Th, 223Ra, and the development of associated radiolabeling methods.
Bioconjugation chemistry
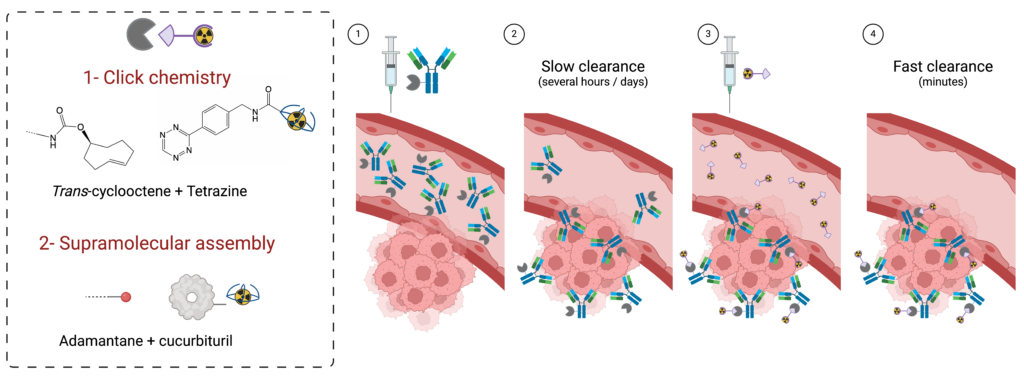
Grafting a chelating agent onto a biovector is known as bioconjugation. This step plays a critical role in the design of radiopharmaceuticals. The chemistry involved must comply with a number of constraints, particularly when applied to complex and fragile biomolecules such as proteins.
The team has developed considerable expertise in the chemical modification of a wide variety of vectors, including antibodies, antibody fragments, peptides and small molecules. The techniques used enable “site-specific” conjugations to be carried out, with precise control over the number and position of modifications made. This know-how is made available to the community via the Bioconjugates Transfer Unit.
We are currently developing platforms for multiple labeling of proteins on a single residue, and tools for pre-targeting approaches using click chemistry or supramolecular chemistry.
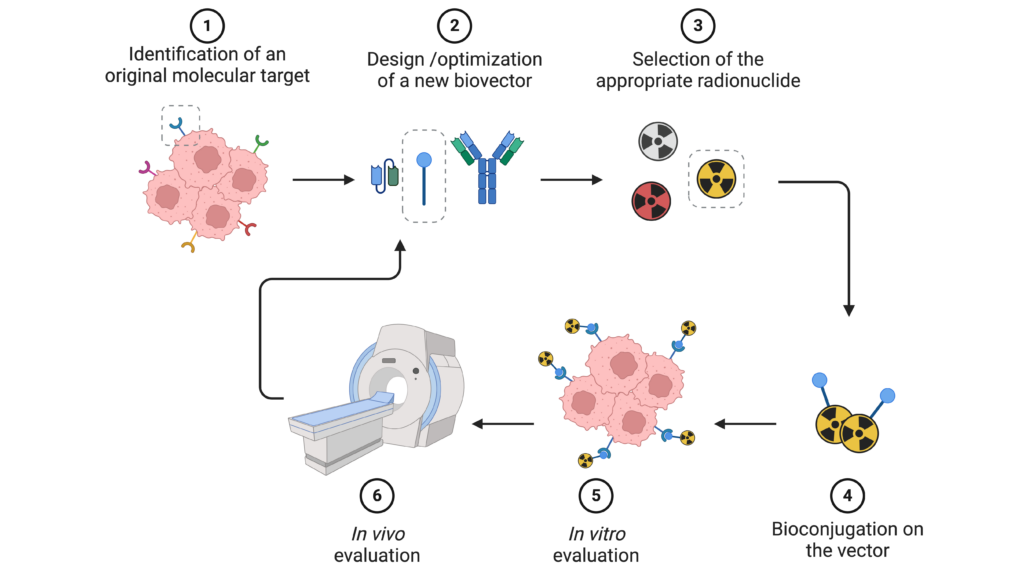
Molecular targeting
In addition to classical vectors (trastuzumab, pertuzumab, PSMA, FAPI, etc.), which we use to validate new molecular tools and methodologies, the team is also interested in validating new pharmacological targets and developing new targeting vectors. The aim is to identify molecular targets that will enable us to meet unmet clinical needs. This work involves the fields of :
– oncology (targeting tumor stroma, hypoxia, etc.), particularly for poor-prognosis cancers such as pancreatic cancer and glioblastoma;
– imaging of fibrosis, in pathologies such as idiopathic pulmonary fibrosis;
– imaging of artherosclerosis.
In-house expertise enables the synthesis and engineering of a wide variety of vectors, from small heterocyclic molecules to full length antibodies. The optimization of these vectors also involves multi-(hetero)valent approaches, in which several vectors are combined in a single compound to increase the affinity and/or selectivity of the radiopharmaceuticals.
Selection of publications
- Moving beyond isothiocyanates: A look at the stability of conjugation links towards radiolysis in 89Zr-labeled immunoconjugates. R. Vizier, P. Adumeau, M. Moreau, V. Goncalves, F. Denat.
Bioconjugate Chemistry (2024), 35, 633–637 - Synthesis and Preclinical Fluorescence Imaging of Dually Functionalized Antibody Conjugates Targeting Endothelin Receptor-Positive Tumors, D. Vivier, M. Hautière, D. Pineau, PA. Dancer, A. Herbet, JP. Hugnot, C. Bernhard, V. Goncalves, C. Truillet, D. Boquet, F. Denat.
Bioconjugate Chemistry (2023), 34, 2144-2153 - SPECT Imaging of Lysyl Oxidase-like 2 in a Model of Idiopathic Pulmonary Fibrosis, R. Vizier, AR. Garnier, A. Dias, M. Moreau, M. Claron, B. Collin, F. Denat, PS. Bellaye, V. Goncalves.
Molecular Pharmaceutics (2023), 20, 3613-3622.
Topic 2 : Radiotheranostics and Multimodality
Find out more about our 3 research topics :
Targeted radionuclide therapy and radiotheranostics
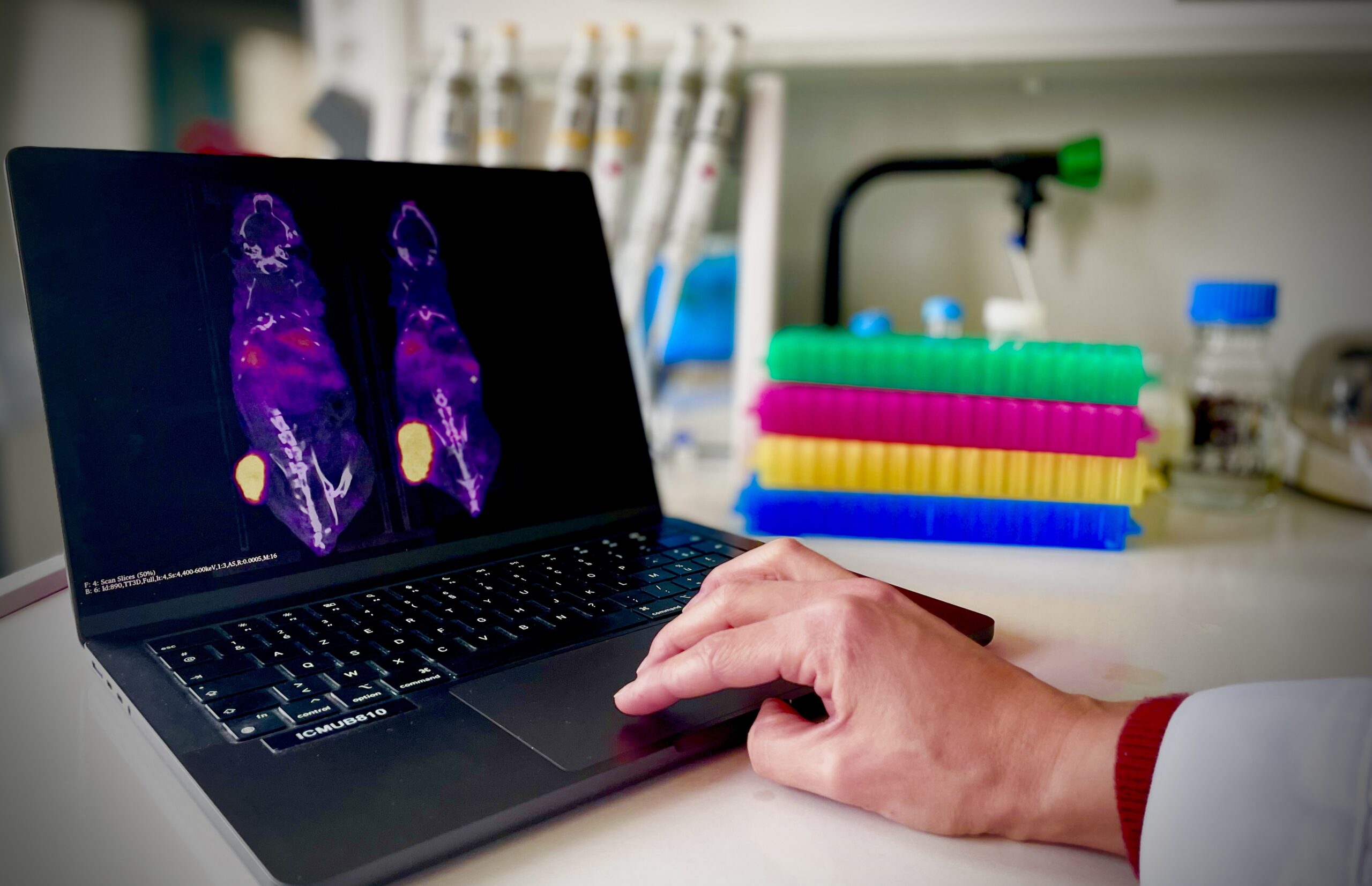
Targeted Radionuclide Therapy (TRT) is playing an increasingly important role in the therapeutic arsenal of oncology. This strategy enables highly selective systemic irradiation of tumors, regardless of their number or location. It relies on the use of a vectorized radiopharmaceutical, specific to a tumor target, carrying a radioisotope whose emitted particles (β-, α- or Auger electrons) are capable of inducing cancer cell death.
Our ambition is to position ourselves in this resolutely interdisciplinary and translational field, thanks to our mastery of the various aspects of the development chain of radiopharmaceutical compounds for TRT, from chemistry to clinical trials.
These projects, by their very nature collaborative, are carried out within consortia involving academic, hospital and often industrial partners. Our programs focus in particular on the development of new TRT molecules for patients with metastatic colorectal cancer (COMETE project) and the study of the combination of TRT with other therapeutic modalities (2in1 ADRC project).
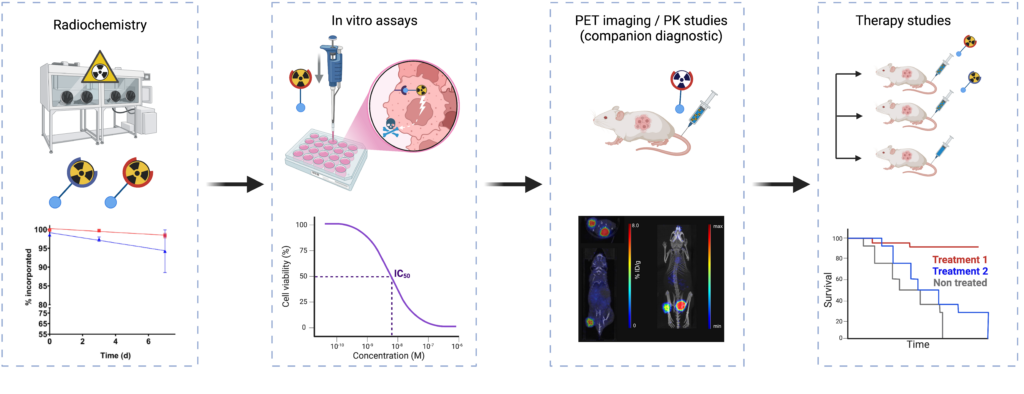
PET/MRI
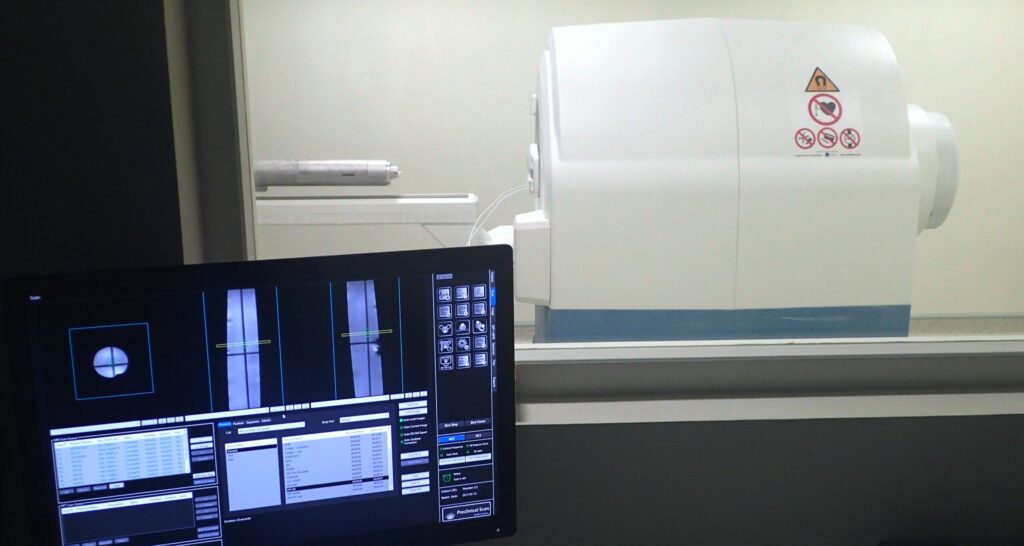
Simultaneous positron emission tomography (PET) and magnetic resonance imaging (MRI) combine two complementary modalities, providing functional and molecular (PET) and anatomical and metabolic (MRI) information.
This synergy has transformed biomedical imaging, both in preclinical research and clinical practice, thanks to its unique integration capabilities. In this context, we have been awarded the EQUIPEX (PIA 1) project IMAPPI (Integrated Magnetic resonance And Positron emission tomography in Preclinical Imaging – ANR-10-EQPX-0005, €7.3m) for the period 2012-2024. The aim of IMAPPI was to develop a simultaneous PET (SiPM solid detectors)-MRI(7 T) imaging instrument, a commercial system since 2017.
This technology represents a major step forward in translational/cross-disciplinary research, particularly in oncology, cardiology, instrumental development and the chemistry of diagnostic agents. In oncology, it combines the molecular sensitivity of PET, enabling detection of metabolic activity and specific receptors, with the anatomical and functional precision of MRI to localize and characterize tumors and metastases, particularly in complex soft tissues. In cardiology, it integrates myocardial metabolic assessment (PET) with detailed structural and perfusion analyses (MRI), optimizing the diagnosis of heart disease. From an instrumental point of view, the integration of these two modalities requires advanced technologies to manage magnetic interference and optimize simultaneous acquisitions for the pathologies we study. Finally, concerning chemistry, the development of paramagnetic contrast agents and specific radiopharmaceuticals enables us to improve the specificity and sensitivity of targeted biomarkers, paving the way for applications in personalized medicine and the exploration of new therapeutic targets.
This multidimensional synergy places PET-MRI at the heart of modern diagnostic innovations, in which our group is particularly active.
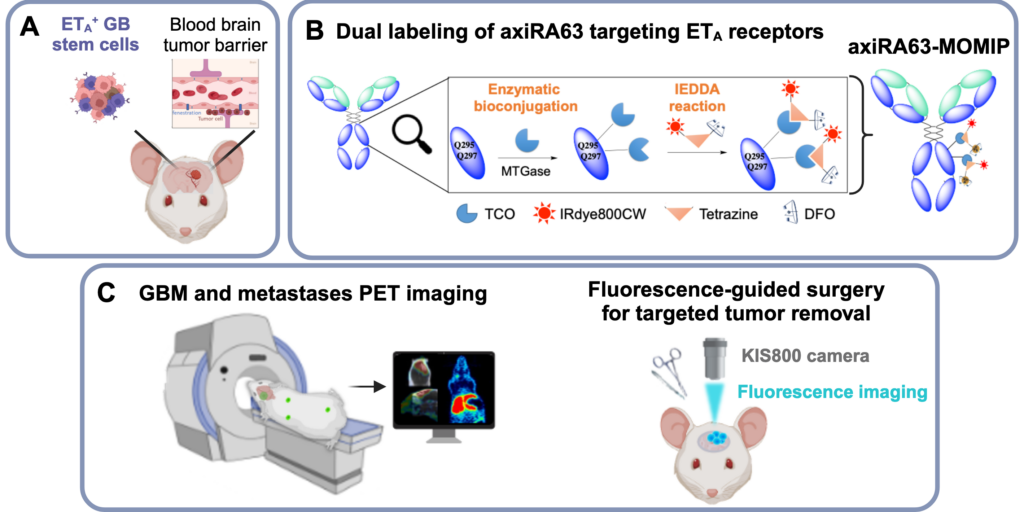
Multimodality : association of nuclear imaging with fluorescence imaging
Similar to PET/MRI imaging, nuclear/optical bimodality aims at combining the advantages of these two techniques, within a single molecule, to improve early diagnosis and therapeutic management of patients. Nuclear imaging (PET or SPECT) is used to detect tumors and any metastases, and to stratify patients. Fluorescence imaging, which is not very penetrating but non-ionizing, improves tumor resection by enabling surgeons to visually distinguish healthy tissue from pathological tissue. The team is designing vectorized probes combining the two modalities, which are being evaluated in preclinical models of poor-prognosis cancers such as glioblastoma and pancreatic cancer.
Selection of publications
- Preoperative PET imaging and fluorescence-guided surgery of human glioblastoma using dual-labeled antibody targeting ETA receptors in a preclinical mouse model: A theranostic approach. M. Hautiere, D. Vivier, P. Dorval, D. Pineau, D. Kereselidze, C. Denis, A. Herbet, N. Costa, C. Bernhard, V. Goncalves, E. Selingue, B. Larrat, P.-A. Dancer, J.-P. Hugnot, D. Boquet, C. Truillet, F. Denat
Theranostics (2024), 14, 6268-6280 - SPECT Imaging of Lysyl Oxidase-like 2 in a Model of Idiopathic Pulmonary Fibrosis. R. Vizier, AR. Garnier, A. Dias, M. Moreau, M. Claron, B. Collin, F. Denat, PS. Bellaye, V. Goncalves.
Molecular Pharmaceutics (2023), 20, 3613-3622. - Modular One-Pot Strategy for the Synthesis of Heterobivalent Tracers. T. Bailly, S. Bodin, V. Goncalves, F. Denat, C. Morgat, A. Prignon, IE Valverde.
ACS Medicinal Chemistry Letters (2023), 14, 636-644
Current projects
We benefit from the support of various regional (FEDER, Graduate Program INTHERAPI, Cancéropole Est), national (ANR, INCA, FLI ) and international (Horizon Europe) agencies and programs. Some of our research is also carried out in partnership with biotech companies in the radiopharmaceuticals field (Oncodesign Precision Medicine, Radiomune Pharma).