CaPCoM
CatalysIS, Polymers, Coordination et MODELLING
Scientific context and objectives
The CaPCoM research group is specialized in the design and synthesis of new metal catalysts based on abundant, non-critical terrestrial elements, from molecular species to innovative metal nanocomposites, for applications in selective catalytic processes. The group is particularly interested in the transformation of bio-based substrates and the production of biodegradable materials, as well as in the production of key compounds for low-carbon energy. Its experimental work draws heavily on her modeling skills, both for understanding mechanisms and predicting properties.
Team members

Team leader: Dr. Raluca MALACEA (CR, CNRS)
- Dr. Julien ROGER (co-leader, MCF)
- Dr. Régine AMARDEIL (MCF)
- Dr. Jacques ANDRIEU (MCF)
- Dr. Jérome BAYARDON (MCF)
- Dr. Audrey BEILLARD (MCF)
- Dr. Gilles BONI (MCF)
- Dr. Laurent BRACHAIS (MCF)
- Dr. Hélène CATTEY (MCF)
- Dr. Virginie COMTE-CANDAS (MCF)
- Pr. Jean-Pierre COUVERCELLE (PU)
- Pr. Paul FLEURAT-LESSARD (PU)
- Pr. Jean-Cyrille HIERSO (PU)
- Pr. Pierre LE GENDRE (PU)
- Dr. Adrien NORMAND (CR, CNRS)
- Dr. Michel PICQUET (MCF)
- Pr. Nadine PIRIO (PU)
- Dr. Laurent PLASSERAUD (CR, CNRS)
- Dr. Sylvie POURCHET (MCF)
- Cédric BALAN (TCH, UBE)
- Dimitri SABAT (IE, UBE)
- Henri SABBADIN (AI, CNRS)
Research topics
Topic 1: Phosphorus chemistry
Find out more about our 3 research topics :
P-chirogenic phosphines
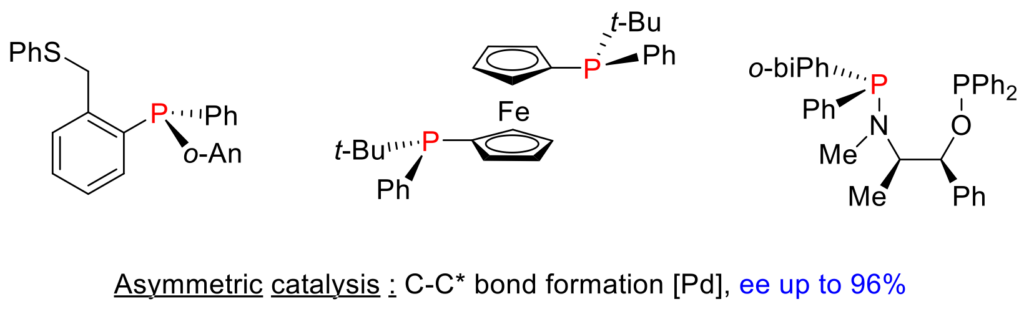
Chiral organophosphorus compounds carriers of the chirality on the phosphorus atom (P-chirogenic) are of interest to agrochemistry, biology and therapeutic chemistry, coordination polymers, the reagents for stereoselective synthesis or asymmetric catalysis and organocatalysis. The interest of chiral organophosphorus compounds comes from the ease to modify their structure and consequently their electronic property and their stereochemistry, by nucleophilic or electrophilic reactions at the the phosphorus center or in the α or β position of a substituent. In our group, we have been interested in the stereoselective synthesis of chiral phosphorus ligands, including ortho-functionalized phosphines, using the ephedrine method or the aryne chemistry. Recently, a new stereospecific rearrangement of aminophosphine boranes into P-chirogenic phosphinites has been developed and has resulted in the formation of P-chirogenic organophosphorus compounds such as aminophosphines-phosphinites (AMPP) and sterically hindered diphosphines which have been applied in asymmetric catalysis in C-C* bond forming reactions with high enantioselectivities.
Team members involved: J. Bayardon, R. Malacea
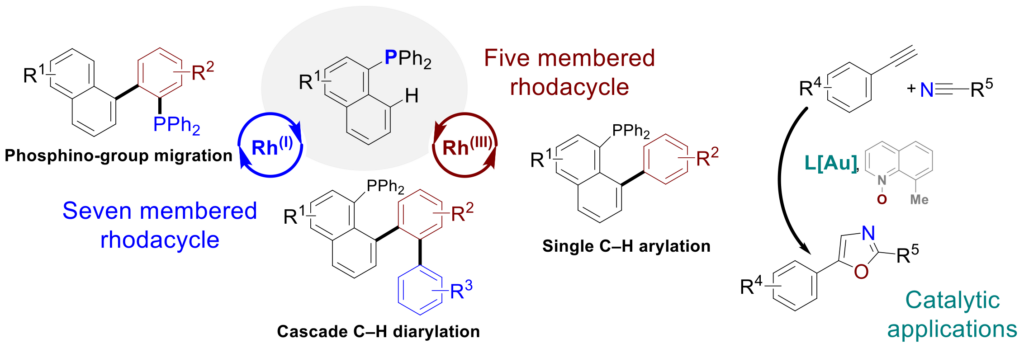
Polyaromatic phosphines (PAH)
Monophosphine polyaromatic hydrocarbons have demonstrated their interest in numerous applications in the fields of coordination, catalysis (such as Buchwald biphenyl-based ligands), health through their analgesic, anti-inflammatory, or anti-cancer activities, as well as in organic electronic materials. These include planar aromatic derivatives such as pyrene, phenanthrene, or fluoranthene, for which their electronic and geometric characteristics can also favor π-stacking interactions. These can be easily obtained and diversified through selective C–H bond functionalizations using phosphorus as a directing group with the help of transition metals such as rhodium (Adv. Synth. Catal. 2021, 364, 440). The nature of the rhodium-based metal precursor RhI vs RhIII can be essential in controlling the functionalization of polyaromatic derivatives. Thus, the cationic precursor [Rh(I)(COD)2] BF4 allows more efficient introduction of bulkier aryl groups, as well as cascade functionalization, or even migration of the phosphorus group via a 7-membered rhodacycle (Eur. J. Inorg. Chem. 2025, e202400721). These (poly)arylated monophosphines have notably improved the catalytic activity of gold in a cyclization reaction towards the formation of oxazoles (Synthesis 2023, 55, 3589).
Team members involved: J. Roger, J.-C. Hierso, P. Fleurat-Lessard, H. Cattey
Ferrocenyl phosphine ligands
The rational design and synthesis of polyfunctional ferrocenyl phosphine ligands, to study their physical properties, their coordination chemistry and their applications in organic synthesis catalysis is very fertile. A wide variety of multifaceted ligands and complexes has thus been created and valorised (Chem. Soc. Rev. 2007, 36, 1754). These new organometallic ligands – stable to air, humidity and temperature – have been used, and commercialized, to catalyse C–C couplings to palladium at reduced metal content (0.1 to 0.0001 mol%), and then for the activation of C–H and C–Cl bonds (Angew. Chem. Int. Ed. 2010, 49, 6650). This homogeneous phase chemistry was transferred in heterogeneous phase to recyclable materials (Chem. Commun. 2014, 50, 9505). Pd catalysis by ferrocenyl phosphine ligands has been examined for C–H alkylation C–C coupling (Angew. Chem. Int. Ed. 2014, 53, 13573), and successfully applied to C–O coupling (Adv. Synth. Catal. 2011, 353, 3403), C–S (Chem. Eur. J. 2014, 20, 12584) and C–N (Catal. Sci. Technol. 2014, 4, 2072). We have addressed synthetic challenges to incorporate into the controlled environment of a ferrocene platform an antagonistic pair of donor (P or N) and electronic acceptor (B) atoms as Lewis pairs (Chem. Commun. 2017, 53, 6017). The range of reactions allowed by these ligands has recently been enriched with the Suzuki reaction to gold (rare), the selective cycloisomerization of enynes (Chem. Eur. J. 2022, e202200769), the nucleophilic fluorination of chloroquinolines by Ag, or the C–H arylation of 1,3,4-oxadiazoles with aryl chlorides at Pd.
Team members involved: J.-C. Hierso, J. Roger, N. Pirio, R. Amardeil, P. Fleurat-Lessard, H. Cattey

Main publications Topic 1
- Phosphorus‐Directed Rhodium‐Catalyzed C–H Arylation of 1‐Pyrenylphosphines Selective at the K‐Region. C. Sire, H. Cattey, A. Tsivery, J.-C. Hierso, J. Roger Adv. Synth. Catal. 2021, 364, 440–452. (10.1002/adsc.202101211) (Highlighted as Hot Topic: C–H activation in Asian JOC).
- Enantioselective Synthesis of P-chirogenic 1,2,3-triazolobenzophospholes. J. Org. Chem. 2024. ⟨hal-04730220⟩, J. Bayardon*, C. Qian, R. Malacea-Kabbara, Y. Rousselin, S. Jugé.
- Highly Functionalized Ferrocenes.E. Lerayer, L. Radal, T. A. Nguyen, N. Dwadnia, H. Cattey, R. Amardeil, N. Pirio, J. Roger, J.-C. Hierso Eur. J. Inorg. Chem. 2020, 2020, 419–445 (10.1002/ejic.201901183)
Topic 2: Coordination chemistry for catalysis

Find out more about our 5 research topics :
CO2 chemistry, bio-based synthons and (de)polymerization
For environmental reasons (depletion of raw materials), economic reasons (price instability) and societal reasons (awareness), the replacement of fossil resources (oil, gas, coal) appears today as a crucial necessity and a real challenge for the 21st century. We are interested in the design of bio-based materials and polymers from carbon dioxide and molecules from biomass to obtain polylactides, polyhydroxy urethanes, polycarbonates or polyepoxides. Carbon dioxide is considered as a chemical raw material in its own right, abundant, of low toxicity and low cost. It can be valorized as a C1 synthon for the synthesis of polymerizable monomers, in particular for the manufacture of polycarbonates and polyurethanes. Various novel complexes based on abundant and biocompatible metals are developed as versatile and robust catalysts for the (co)polymerization of bio-sourced monomers. The end-of-life of materials and their depolymerization for recycling purposes are also taken into account by developing suitable metal catalysts and, if necessary, by using alternative solvents or bio-sourced ligands.
Team members involved: L. Plasseraud, P. Le Gendre, R. Malacea, J. Bayardon, J. Andrieu, M. Picquet, G. Boni, J. P. Couvercelle
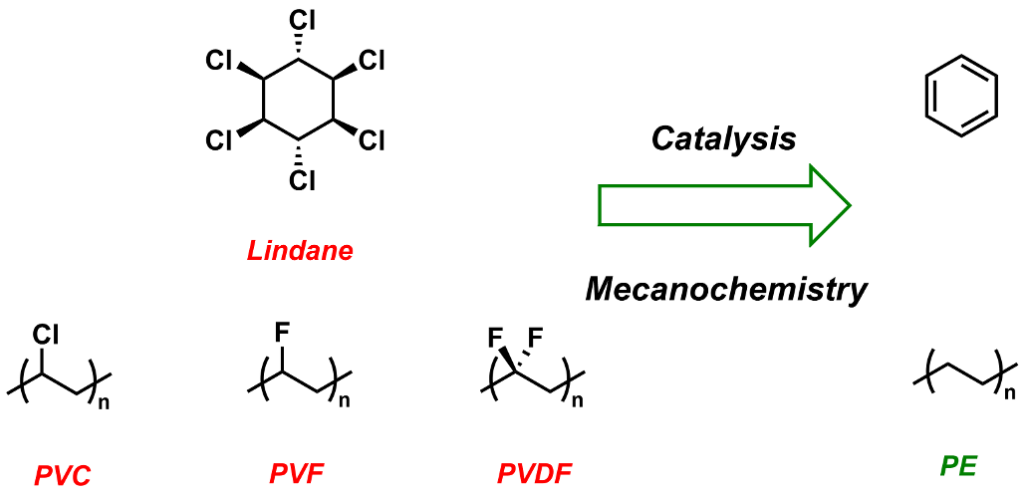
Dehalogenation of polymers and persistent organic pollutants (POPs)
Halogenated substances can be found in a great number of consumer items, building materials and pesticide formulations. For various reasons (toxicity, POP character, presence of “legacy” additives), the end-of-life management of these substances is troublesome: on the one hand, there is a lack of technically and economically viable recycling processes, and on the other hand destruction by incineration is prohibited because of the release of vast quantities of acids (HCl or HF), which can corrode industrial plants. In order to tackle this challenge, we have recently reported a methodology for the zirconium-catalyzed dechlorination of PVC in the presence of silanes under mild conditions. We are currently extending the scope of this methodology to other substrates (lindane, fluorinated polymers, PFAS…) and we are developing new methodologies (titanium catalysis, mecanochemistry) in collaboration with LCC Toulouse and LCPO Bordeaux, or internally. Funding for this research is provided by CNRS (Emergence@INC scheme) and ANR (DEHAYLIDES project).
Team members involved: A. Normand, A. Beillard
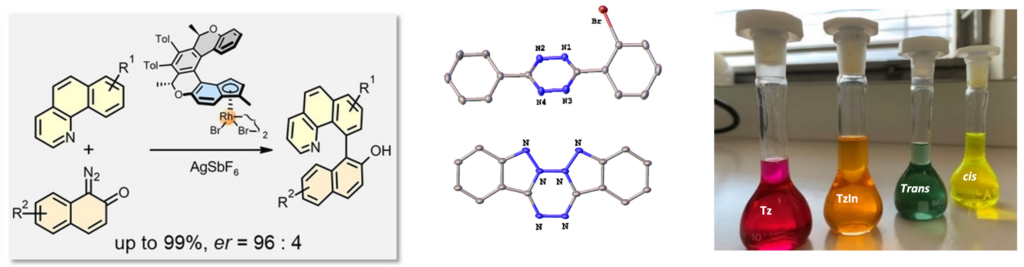
CH activation, (poly)-nitrogen-containing systems
(Poly)Nitrogen-containing heterocycles are important compounds for numerous applications, ranging from their use as ligands in organometallic chemistry, to their presence in agrochemistry, healthcare, and molecular materials. More selective, faster and greener synthesis methods are therefore a major challenge for our society. One possible strategy is the selective C–H bonds activation in the ortho-position (or more distant) of the nitrogen core by using the nitrogen atom as a directing group via the transient formation of a metallacycle (Pd, Ru, Ir, Rh, etc.). This methodology has been used to obtain chiral benzoquinolines via the asymmetric arylation of benzoquinoline with diazonaphthoquinones catalyzed by chiral helicene-indenyl rhodium complexes. (Angew. Chem. Int. Ed. 2025, 64, e202421512). It has also enabled the development of an innovative synthetic route for polyfunctionalized s-aryltetrazines compared to traditional methods (Pinner or Stollé) (Angew. Chem. Int. Ed. 2016, 55, 5555). This new synthesis method has led to the emergence of new applications for this type of molecule, such as doubly “clickable” polyazotic systems (via IEDDA coupling or Huygens coupling; Angew. Chem. Int. Ed. 2020, 59, 1149) or optically active molecules like the bis-tetrazolo[1,2-b]indazoles (Angew. Chem. Int. Ed. 2023, 62, e202300571), and has also allowed for the rapid and efficient expansion of the family of polyhalogenated s-tetrazines, whose fungicidal properties are well established (Clofentezine®, ACS Catal. 2017, 7, 8493).
Team members involved: N. Pirio, J.-C. Hierso, J. Roger, A. Beillard
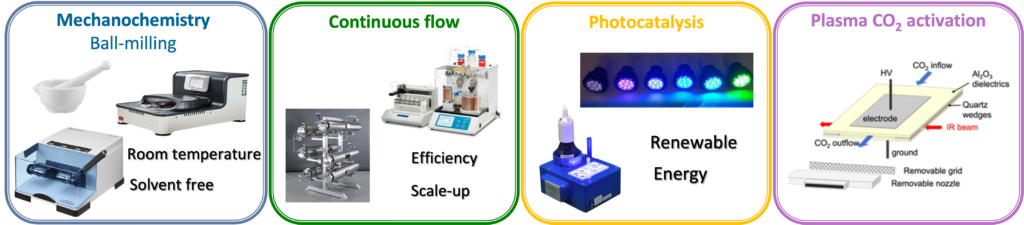
Innovative activation methods : photocatalysis, mechanochemistry, plasma
For several years, researchers have been aiming to improve organic synthesis processes to make them more efficient, faster, and to access new reactivities. In this sense, various technologies or activations have been developed:
- Mechanochemistry, which relies on mechanical activation and has the secondary advantage of working with a minimum amount of solvent;
- Photocatalysis, which uses a catalyst able to absorb light energy and to transfer it to a reaction, allowing access to new synthesis pathways and new products;
- The use of microwaves, continuous flow, which, by their ability to heat a reaction mixture more locally and efficiently, reduces reaction times and improves reactivity;
- Plasma activation of strong bonds in cooperation with physicists in the framework of a joint program combining mechanistic study and synthetic applications.
All these innovative technologies and activations are used in our team for various applications, including (photo)-catalysis or the synthesis of catalysts for the development of new processes considered as more eco-compatible, and allowing the activation of more difficult bonds (C–H) and molecules (CO2) by traditional thermal approaches.
Team members involved: A. Beillard, J.-C. Hierso, N. Pirio
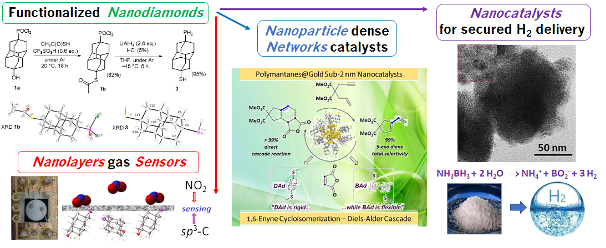
Nanocatalysis and nanochemistry (nanodiamonds, nanoparticles, H2)
We study the bottom-up construction of original nanostructures ordering, in different ways, purely organic functionalized nanodiamonds and transition metals, to pave the way for novel hybrid materials. Potential applications include energy transition and catalytic processes, and aim to exploit diamond’s exceptional electronic and robustness properties at the nanoscale. “Molecular” nanodiamonds (diamondoids) that include the higher homologues of adamantane (diamantane, bisadamantane, etc.), represent a singular form of carbon-sp3 close to diamond materials at the nanometer scale. They are distinct from, and complement carbon-sp2 derivatives such as fullerenes, graphenes and CNTs. We have described an innovative dry methodology (gaseous: PVD and CVD) for the fabrication of ultrafine palladium and gold nanolayer composite materials over structured diamondoid self-assemblies (Adv. Funct. Mater. 2018, 1705786). The nanocomposites formed are reversible gas sensors (NO2, H2) that are very sensitive at low temperatures (Angew. Chem., Int. Ed. 2019, 58, 9933). In solution, functionalized diamondoids stabilize nanoparticles rather than layers, forming ultra-selective dispersed nanocatalysts for the cycloisomerization of enynes with gold (J. Am. Chem. Soc. Au., 2021, 1, 187). The synthesis of nanocatalysts for H2 destocking from solid sources (amine borane) –a safer solution than H2 under pressure or cold– is based on Ru and Ni. Our approach is closely associated with the trajectory of our partners (LCC CNRS Toulouse, M. Kahn group) aiming at the design and development of nanoobjects and complex nanomaterials finely characterized, including by the tools of theoretical chemistry.
Team members involved: J.-C. Hierso, N. Pirio
π-Conjugated Polyaza Ligands: Coordination Chemistry and Catalysis
Conjugated polyaza ligands, like amidines have received limited attention in the fields of coordination chemistry and catalysis. However, they are potentially better ligands than Schiff bases, given their conjugated character. In this context, we have developed a new family of ligands by replacing the imine motif of salicylaldimine ligands (phenoxy-imine) or bis(salicylaldimine) ligands (Salen) with an amidine function (ANR MORFAL). NMR, XRD, and DFT studies have identified unique features in amidine coordination modes (end-on/side-on/η3 coordination, fluxional character, hemilability…). Zinc or aluminum complexes with phenoxy-amidine ligands have been demonstrated to be highly effective in ring-opening polymerization (ROP) of lactide (Organometallics 2019, 38, 4147, ibid. 2022, 41, 2920, Eur. Pat. 22305933.8, 2022, Dalton Trans. 2023, 52, 7854). Titanium complexes with chiral phenoxy-amidine ligands derived from prolinol have been used in the asymmetric silylcyanation of aldehydes (Eur. J. Inorg. Chem. 2024,27, e202400405). Bis(phenoxy-amidine) ligands (FAlen) were developed, and their lanthanum complexes were shown to promote the ROP of butyrolactones with very high isotacticity (Pm up to 0.93). (Inorg. Chem. 2023, 62, 7342, Angew. Chem. Int. Ed. 2025, e202509587). In collaboration with Prof. Aleš Růžička’s group at the University of Pardubice, we studied conjugated polyaza biguanide ligands. Biguanide zinc complexes exhibited exceptional activity in ROP of lactide at room temperature (ACS Catalysis 2025, 15, 9117).
Team members involved: P. Le Gendre, R. Malacea, J. Bayardon, C. Balan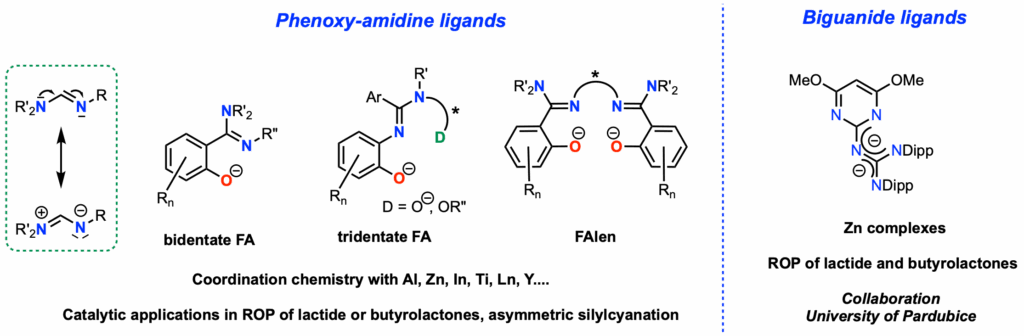
Main publications Topic 2
- Can supercritical carbon dioxide be suitable for the green pretreatment of plant fibres dedicated to composite applications ? C. François, V. Placet, J. Beaugrand, S. Pourchet, G. Boni, D. Champion, S. Fontaine, L. Plasseraud, J. Materials Science, 2020, 55, 4671–4684
- Bis(salicylamidine) Ligands (FAlen): A Variant of Salen with “à la Carte” Denticity. V. Vaillant-Coindard, F. Chotard, B. Théron, C. Balan, J. Bayardon, R. Malacea-Kabbara, E. Bodio, Y. Rousselin, P. Fleurat-Lessard, P. Le Gendre, Inorg. Chem. 2023, 62, 19, 7342–7352
- Stereocontrolled Synthesis of Chiral Helicene-Indenido ansa- and Half-Sandwich Metal Complexes and Their Use in Catalysis. T. Edlová, J. Rybáček, H. Cattey, J. Vacek, L. Bednárová, P. Le Gendre, A. T. Normand, I. G. Stará, I. Starý, Angew. Chem. Int. Ed. 2025, 64, e202421512 (10.1002/anie.202421512).
- Tetrazo[1,2-b]indazole: Straightforward Access to Nitrogen-Rich Polyaromatics from s-Tetrazines. A. Daher, A. Bousfiha, I. Tolbatov, C. D. Mboyi, H. Cattey, T. Roisnel, P. Fleurat-Lessard, M. Hissler, J.-C. Hierso, P.-A. Bouit, J. Roger, Angew. Chem. Int. Ed. 2023, 62, e202300571 (10.1002/anie.202300571).
- Poly(vinyl chloride) Dechlorination Catalyzed by Zirconium. A. T. Normand*, Y. Wu, T. Régnier, P. Fleurat-Lessard, Y. Rousselin, B. Théron, P. Le Gendre et M. Carta, Chem. Eur. J. 2024, 30, e202304005.
Topic 3: Structure and modelling

Find out more about our 3 research topics :
Mechanistic understanding, structures, reactivity, and interactions
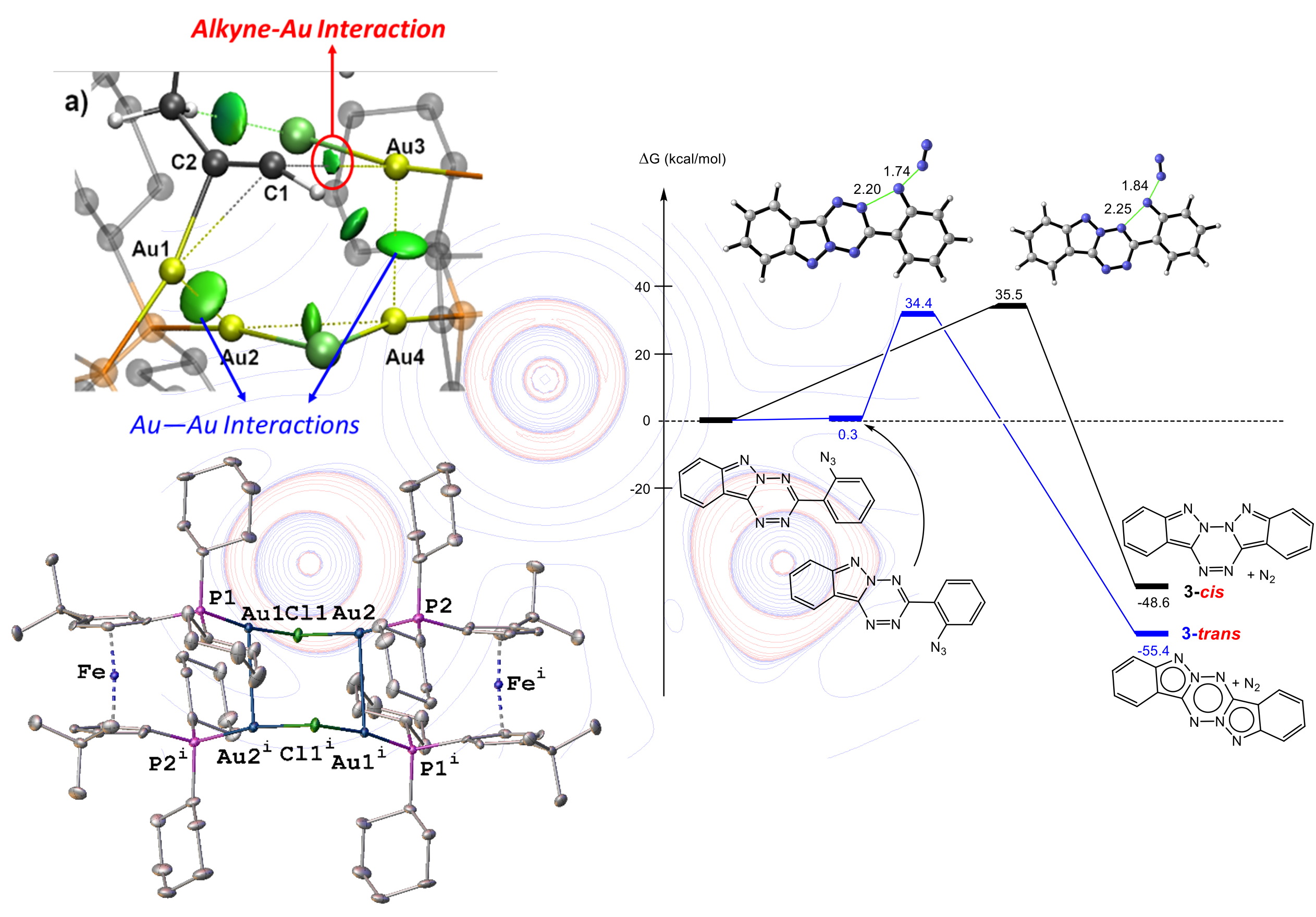
Transition metal-catalyzed reactions constitute a fundamental family in synthetic organic chemistry. Theoretical chemistry and X-ray diffraction play a crucial and complementary role in understanding and developing new organometallic catalysts, particularly through the detailed analysis of interactions between the metal, the ligand, and the substrates. These two complementary methods rely on the same object: electron density. For the resolution of crystal structures, this density modulates the intensity of diffraction peaks and thus allows us to trace the 3D structure to the atomic level for a better understanding of reaction mechanisms and the determination of geometric parameters (interactions, stacking, etc.). In quantum chemistry, electron density allows us to calculate all the properties of molecules: their geometries, their electronic properties, etc. In particular, topological approaches such as Atoms In Molecules (AIM, by R. Bader), Electron Localization Function (ELF) or the Conceptual Density Functional Theory (developed by R. Parr) allow us to “translate” the electronic structure of complexes into chemical concepts such as dative bonds, covalent bonds, mesomeric forms, etc., but also electrophilic or nucleophilic sites to predict their reactivity. In addition, calculating the energies of intermediates and transition states leads to the reaction mechanism. To facilitate the search for transition states in these flexible and complex systems, we have developed the Opt’n Path program, freely distributed and interfaced with several quantum chemistry programs.
Team members involved: P. Fleurat-Lessard, H. Cattey
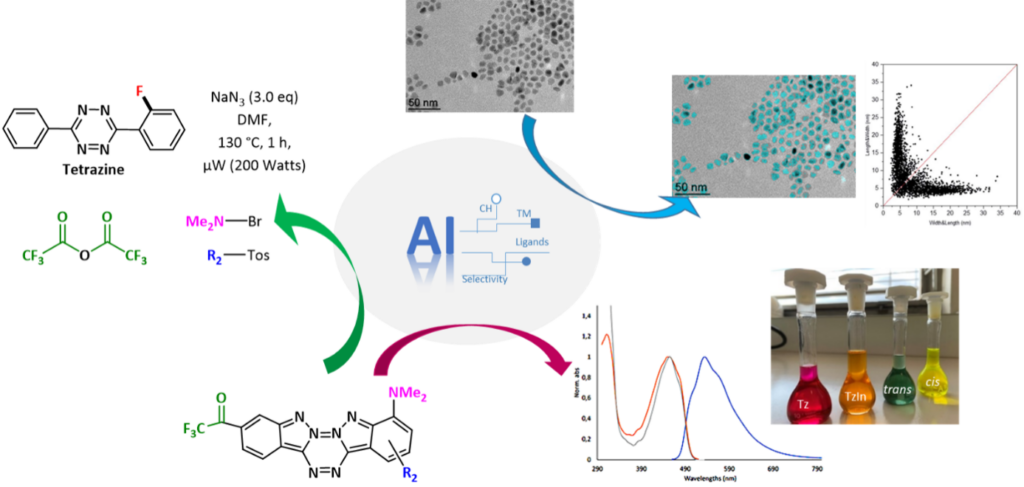
Artificial intelligence: analyze images, spectroscopic simulations
Artificial intelligence (AI) has been used in chemistry for over 30 years, but the rise of deep learning has revolutionized the field. These approaches now offer promising opportunities to optimize reaction conditions, design molecules through AI-assisted retrosynthesis, analyze images, and predict physicochemical properties. We are currently developing AI-based tools for the analysis of transmission electron microscopy (TEM) images. The goal is to (semi-)automatically identify and measure nanoparticle sizes. These data are then processed statistically, notably through 2D plots, to correlate the obtained populations with experimental conditions. AI is also employed to optimize reaction conditions in two major families of reactions selected based on the laboratory’s expertise: (i) the direct C–H activation functionalization of molecules and (ii) the synthesis of nanoparticles. A key aspect of this project is the construction of a well-characterized reaction database. We are now working on automatically populated databases through AI-assisted extraction of data (reactants, products, experimental conditions) from scientific articles and patents. Furthermore, we are exploring AI for predicting the optical properties of molecules developed in our lab, such as (aza)-BODIPY, to streamline synthesis efforts. Finally, we are working on AI-driven interatomic potential adjustments to accelerate molecular simulations, allowing for more accurate solvent inclusion and enabling the study of more complex systems.
Team members involved: . Fleurat-Lessard, J.-C. Hierso, J. Roger
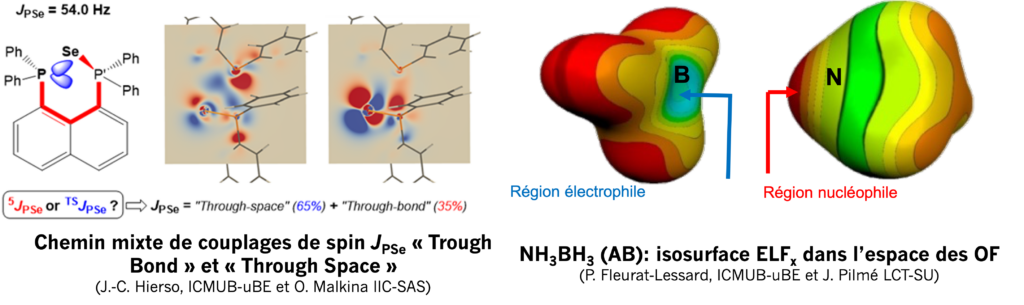
Theory of magnetic spin coupling and chemical bonding
The power of nuclear spin magnetic resonance (NMR) is indispensable for the determination of new molecular structures in inorganic, organic, and biological compounds, or for medical imaging for diagnosis and care. Despite this maturity, the phenomenon of spin coupling (identified by the coupling constant xJAB) and its mode of transmission –seen through the “classical” theory of chemical bonding– remains poorly understood (Chem. Rev. 2014, 114, 4838). Our overall goal is to extend the current perception and understanding of nuclear spin coupling as one of the essential NMR parameters for the characterization of molecules. We use experimental models of polyphosphines (J. Am. Chem. Soc. 2004, 126, 11077) and advanced theoretical tools (J. Am. Chem. Soc. 2022, 144, 10768). New spin-coupling pathways are discovered, explored, and rationalized by visualization modelling (Phys. Chem. Chem. Phys. Phys. 2022, 24, 24039). This fundamental program should have an impact on the interpretation of NMR phenomena, magnetic spin coupling, and on the overall understanding of the nature of chemical bonding. In this vein, we study the reactivity of the ELF localization function and the “Non-Covalent Index” within the framework of topological approaches to chemical bonding. This makes it possible to quantify the role of the different weak and strong interactions along reaction coordinates. The objective is to identify the reactive sites of a molecule in the field of conceptual DFT by identifying and exploiting descriptors capable of evaluating global or local reactivity.
Team members involved: J.-C. Hierso, P. Fleurat-Lessard
Main publications Topic 3
Current projects
We have the support of various regional (including the EIPHI Graduate Program), national (ANR PRC, France 2030, Plan de Relance, CNRS Innovation) and international agencies and funding schemes.